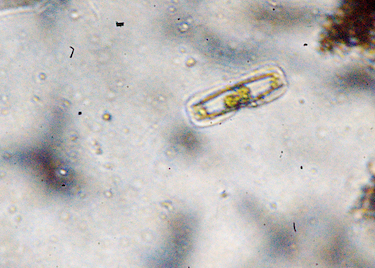
Diatom biodiversity
Diatoms are a major group of algae, and are among the most common types of phytoplankton.
Diatoms are a widespread group and can be found in the oceans, in freshwater, in soils and on damp surfaces.
Diatom communities are a popular tool for monitoring environmental conditions, past and present, and are commonly used in studies of water quality, by studying their colonies and diversity of species.
This is a working protocol to visualize the diatoms in your local waters.
As a workshop, plan for at least 2 days.
Background
Diatoms are a major group of algae, and are among the most common types of phytoplankton. They are photosynthetic and are orange-ish green in colour because of the presence of caroteniods along with chlorophyll. Most diatoms are unicellular, although they can exist as colonies in the shape of filaments, ribbons, zigzags, fans etc. They are generally microscopic. A unique feature of diatom cells is that they are enclosed within a cell wall made of silica (hydrated silicon dioxide) called a frustule. These frustules show a wide diversity in form, but are usually almost bilaterally symmetrical, hence the group name. The symmetry is not perfect since one of the valves is slightly larger than the other allowing one valve to fit inside the edge of the other. Diatom communities are a popular tool for monitoring environmental conditions, past and present, and are commonly used in studies of water quality, by studying their colonies and diversity of species.
Diatoms are a widespread group and can be found in the oceans, in freshwater, in soils and on damp surfaces.
Materials
- Plastic tray
- Toothbrush with hard bristle preferably
- Drain cleaner (Anything containing sodium hypochlorite, Domex in India, Domestos Indonesia )
- Bigger eppendorf tubes, which can be used in a suitable centrifuge
- Test tubes and stand
- Pipet
- Plastic pipet/dropper
- A device which shows gps coordinates – smart phone in our case (GPS test app)
- PVLG mounting medium (optional, see recipe below)
PVLG Receipe
PVLG will allow for more stable sample viewing
Materials:
- 100 mL distilled water
- 100 mL lactic acid
- 10 mL glycerol
- 16,6 g polyvinyl alcohol (PVA)
Preparation:
- To prepare PVLG, add the polyvinyl alcohol (a dry powder) to the water and put in 60 degree oven until dissolves.
- Add lactic acid and glycerine and allow the solution to set for 24 hours before first using.
Use:
- Specimens can be mounted directly in the PVA solution, or the solution can be added to the sides of the cover slips thatwere made with water, lactophenol, or melzer’s reagent.
- The PVA solution will infiltrate the material in a day or two.
- Slides made with PVLG can be hardened by heating at 40-75oC overnight.
- Immersion oil can be wiped from these hardened slides without disturbing the specimen.
Equipment
- microscope, modified hackteria-style webcam microscope: with increased distance between the lens and the sensor to increase magnification. We need a minimum magnification of around 300-400X
- OPTIONAL but highly recommended - microcentrifuge to separate the diatoms, such as the one from GaudiLabs, or the dremel centrifuge
Methods
- Identify the water body and location from where the diatoms are to be sampled. Mark the coordinates on the gps.
- Choose a rock/stone which is flat, covered by the water and is at a shallow part of the water, which receives enough sunlight. We chose the ones which were close to the shore/bank.
- Pick up the rock. It should feel slimy and have an orange-ish green tinge (maybe just in parts, but that is fine). If it is slimy, place it into the tray or else choose another rock.
- Scrub the rock using the toothbrush to remove all the diatoms. Using a dropper, pour some clean water on the rock. The orange-ish water collected in the tray contains the diatoms.
- For a simple observation, just take a drop of this water and observe under the microscope.
- To isolate the diatoms from other micro algae, samples of the water from the tray can be centrifuged and observed. The top layer would have the highest concentration of diatoms. In the absence of a centrifuge, the water can be placed in a test tube, swirled once and then left on a stand till it settles. The top layer here too has the highest concentration of the diatoms.
The diatoms are alive here and some species are moving, which can make them hard to observe. If the concentration of the diatoms is high, this sample on the slide can be dried. After it has dried, The slide can be preserved using several methods. We used Akbars #PVLG Receipe and after mounting, sealed the slide with transparent nail polish.
To observe just the silica casing of the diatoms continue to the following.
This process of extraction of the Diatoms takes around 1 to 1.5 hours.
- Take the water from the tray containing the diatoms and put it into eppendorf tubes and centrifuge.
- Once all the algae is in the bottom, using a dropper, remove all the water without disturbing the diatom and algae layer.
- Fill the tube with drain cleaner, and mix it through all the layers. Let this solution stand for half an hour, and agitate it 3 to 4 times while it sits.
- After half an hour centrifuge the tubes again. This time, there should be a white layer which forms between the liquid and muddy layer. This White layer consists of the diatoms. Before observing this, they need to be cleaned of the drain cleaner though.
- To clean the diatoms, remove the drain cleaner without disturbing the other layers and add clean water to the tubes. Mix it well and centrifuge. Once the layers are separate, again replace the water with some fresh water and repeat the process. This needs to be done around 3 times.
- After the final wash, the water can be thrown. The diatoms can be carefully removed from the white layer using a pipette and observed under the microscope. They can be mounted using Akbars PVLG recipe.
References Documentation
- biodesign.cc entries - Isolation Process, Workshop in HLab14 Yogyakarta, sampling in Arunachal Pradesh
- California Academy of Sciences DIATOM COLLECTION
- Malviya S, Scalco E, Audic S, et al. Insights into global diatom distribution and diversity in the world’s ocean. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(11):E1516-E1525. doi:10.1073/pnas.1509523113. (ncbi link)
- Data companion website from article - "Eukaryotic plankton diversity in the sunlit ocean". First Tara Oceans V9 rDNA metabarcoding dataset.
- Diatoms page on the Tree of Life Web Page project.