Difference between revisions of "Circular chromatography"
(→Step by Step) |
(→Introduction) |
||
| (24 intermediate revisions by 2 users not shown) | |||
| Line 4: | Line 4: | ||
[http://www.dictionary.com/browse/graphy?s=t GRAPHY] = writing...representing...or a process, or art / science working with such a process | [http://www.dictionary.com/browse/graphy?s=t GRAPHY] = writing...representing...or a process, or art / science working with such a process | ||
| − | Chromatography is a way to analyse what is in a mixture (in this case liquid) - by separation on solid support (in this case paper), and visualization in this case, by a [https://en.wikipedia.org/wiki/Photographic_processing photographic process].<br> | + | Chromatography is a way to analyse what is in a mixture (in this case liquid) - by separation on solid support (in this case the fibers in the paper), and visualization in this case, by a [https://en.wikipedia.org/wiki/Photographic_processing photographic process].<br> |
"The mixture flows though a medium and the individual substances that make up the mixture are deposited at different distances from the point of inflow." - from website of [http://biodynamics.in/chrom.htm Bio-dynamic Society of India] | "The mixture flows though a medium and the individual substances that make up the mixture are deposited at different distances from the point of inflow." - from website of [http://biodynamics.in/chrom.htm Bio-dynamic Society of India] | ||
| − | + | The separation happens as the liquid slowly wets the paper by [https://en.wikipedia.org/wiki/Capillary_action capillary action] - <br> | |
| + | Those compounds that stick to the paper (medium/ '''stationary / solid phase'''), will stay closer to the point of inflow.<br> | ||
| + | Those compounds that prefer the liquid ('''solvent''', '''mobile phase'''), it will travel further with the liquid.<br> | ||
| + | This process can just be called [https://en.wikipedia.org/wiki/Paper_chromatography paper chromatography]. Because we use paper as a support.Because we did this on a circular piece of paper, it is called circular chromatography. <br> | ||
| + | |||
<br> | <br> | ||
In this case, we took some soil and mud samples we had collected in different water bodies locations, and tried to analyse the humic content of the samples.<br> | In this case, we took some soil and mud samples we had collected in different water bodies locations, and tried to analyse the humic content of the samples.<br> | ||
| − | This method can be applied to any other liquid mixtures.<br><br> | + | This method can be applied to any other liquid mixtures.<br> |
| + | For example, [https://www.google.co.in/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwizscXL-qDTAhXEpo8KHdVgC-8QFggkMAA&url=http%3A%2F%2Fjournal.iisc.ernet.in%2Findex.php%2Fiisc%2Farticle%2Fdownload%2F2786%2F4173&usg=AFQjCNE4N72bxFl2hwdMwSm47mFqaQ9xZw&sig2=ikNpi2l8nfCn-YukCpOseA&cad=rja this 1953 paper] uses butanol-based solvents to separate different sugars, and visualizes the different sugars. <br> | ||
| + | [https://en.wikipedia.org/wiki/Benedict%27s_reagent Benedict's reagent] may be a nice DIY mix for looking for reducing sugars.<br><br> | ||
| + | |||
| + | To learn more, here is the Khan Academy's explanation of the [https://www.khanacademy.org/test-prep/mcat/chemical-processes/separations-purifications/a/principles-of-chromatography Principles of Chromatography]. | ||
| + | <br><br> | ||
==Methods== | ==Methods== | ||
| Line 25: | Line 34: | ||
===Materials=== | ===Materials=== | ||
---- | ---- | ||
| − | '''Chemicals''' | + | '''Chemicals''' (!! again, this depends on what you want to separate, and what compounds you want to see) |
| − | * 0.5% solution of silver nitrate (you can find in photography suppliers) | + | * '''for visualization''' - 0.5% solution of silver nitrate (you can find in photography suppliers) |
| − | * 0.1 to 1% solution of [https://en.wikipedia.org/wiki/Sodium_hydroxide sodium hydroxide] (NaOH, otherwise known as lye or caustic soda - be careful, because it can burn) | + | * '''solvent, mobile phase''' - 0.1 to 1% solution of [https://en.wikipedia.org/wiki/Sodium_hydroxide sodium hydroxide] (NaOH, otherwise known as lye or caustic soda - be careful, because it can burn) |
'''Other materials''' | '''Other materials''' | ||
* paper (Whatman, or any acid-free thick paper) | * paper (Whatman, or any acid-free thick paper) | ||
| Line 50: | Line 59: | ||
::* 0.2% NaOH in water | ::* 0.2% NaOH in water | ||
::* isopropyl alcohol | ::* isopropyl alcohol | ||
| − | <br> | + | To learn more, here is a [https://www.khanacademy.org/test-prep/mcat/chemical-processes/separations-purifications/v/extractions Khan Academy video on extractions].<br> |
| + | !!!!Dilute NaOH is used to maximize the [http://karnet.up.wroc.pl/~weber/ekstrak2.htm recovery of humic substances from soil]. For analysis of other compounds, make sure to use appropriate extraction liquid. | ||
| + | <br><br> | ||
<p> | <p> | ||
# Take one spoon scoop of soil/mud into petridish to mix and extract with NaOH.<br> | # Take one spoon scoop of soil/mud into petridish to mix and extract with NaOH.<br> | ||
| Line 66: | Line 77: | ||
<br> | <br> | ||
'''DAY2'''<br> | '''DAY2'''<br> | ||
| − | The next steps needs to happen in '''a darkroom''' with a red lamp.<br> | + | The next steps needs to happen in '''a darkroom''' with a red lamp.<br><br> |
| + | <gallery> | ||
| + | File:CC_silvernitrate.png|Preparation of light sensitive paper | ||
| + | File:CC_sample_application.JPG|Sample application | ||
| + | File:CC_exposure_to_light.JPG|Exposure to light | ||
| + | </gallery> | ||
| + | |||
====Preparation of Light sensitive paper==== | ====Preparation of Light sensitive paper==== | ||
| − | |||
# Make the 0.5% silver nitrate solution | # Make the 0.5% silver nitrate solution | ||
# Place 2mL of the 0.5% silver nitrate solution in a clean container | # Place 2mL of the 0.5% silver nitrate solution in a clean container | ||
| Line 76: | Line 92: | ||
# Remove the wick | # Remove the wick | ||
# Let the filter paper dry. | # Let the filter paper dry. | ||
| − | + | ||
====Sample application==== | ====Sample application==== | ||
| − | |||
# Add a new wick on the prepared paper above | # Add a new wick on the prepared paper above | ||
| + | |||
| + | [[File:IMG 8929-wix and filter paper.JPG|640px]] | ||
| + | |||
# Take 2mL the liquid part of the samples prepared above, and place in a cleaned container | # Take 2mL the liquid part of the samples prepared above, and place in a cleaned container | ||
| + | |||
| + | [[File:Wik and solution.JPG|640px]] | ||
| + | |||
# Let the sample travel til just before the silver nitrate circle | # Let the sample travel til just before the silver nitrate circle | ||
| + | |||
| + | [[File:Dipping sample.JPG|640px]] | ||
| + | |||
# Remove wick | # Remove wick | ||
| + | |||
| + | [[File:Dipped paper.JPG|640px]] | ||
| + | |||
# Let the filter dry | # Let the filter dry | ||
| − | + | ||
====Exposure to see the different bands==== | ====Exposure to see the different bands==== | ||
| − | |||
# Take the filters and expose to indirect light, or in more controlled situations, use a timer and a light set up | # Take the filters and expose to indirect light, or in more controlled situations, use a timer and a light set up | ||
# et voila! time for analysis | # et voila! time for analysis | ||
<br> | <br> | ||
| + | |||
| + | ===Analysis of the Chromatogram=== | ||
| + | The analysis of the final chromatogram - the filter paper - seems more difficult.<br> | ||
| + | Comparison between before and after, different locations, may be easier than to pin-point exactly what is producing the patterns.<br> | ||
| + | See one explanation at the bottom of this [http://www.biodynamics.in/chrom.htm webpage]. | ||
| + | <br> | ||
| + | The different colours we obtain has to be a reflection of the [http://www.saltlakemetals.com/Silver_Nitrate_Uses.htm chemical reaction with silver nitrate] and whatever compound that is deposited there. (It seems to be copied from [https://www.scrapmetalforum.com/scrap-metal-tips-advice/13866-silver-what-used-most-useful-all-noble-metals.html here]) | ||
| + | <br><br> | ||
| + | For Halides<br> | ||
| + | For example: <br> | ||
| + | * White=Chloride | ||
| + | * Pale Yellow=Bromide | ||
| + | * Yellow=Iodide. | ||
| + | Test for the presence of Carbonate, Hydroxide, Sulfide and Phosphate ions.<br> | ||
| + | For example: <br> | ||
| + | * Pale Green=Carbonate | ||
| + | * Brown=Hydroxide | ||
| + | * Black=Sulfide. | ||
| + | <br><br> | ||
===Which method to use=== | ===Which method to use=== | ||
| Line 110: | Line 155: | ||
#[https://ledepotesta.wordpress.com/2016/01/20/koliskos-agriculture-of-tomorrow-pt-2/#more-133 Summary of Kolisko's book] | #[https://ledepotesta.wordpress.com/2016/01/20/koliskos-agriculture-of-tomorrow-pt-2/#more-133 Summary of Kolisko's book] | ||
#[http://www.sciencegroup.org.uk/history.htm History of Science Group of the Anthroposophical Society in Great Britain] | #[http://www.sciencegroup.org.uk/history.htm History of Science Group of the Anthroposophical Society in Great Britain] | ||
| − | + | #[https://www.khanacademy.org/test-prep/mcat/chemical-processes/separations-purifications/a/principles-of-chromatography Khan Academy Principles of Chromatography] | |
| + | #[http://karnet.up.wroc.pl/~weber/ekstrak2.htm Extraction of Soil Organic Matter] | ||
| + | #[http://lab-training.com/2014/11/21/types-paper-chromatography/ Types of Paper Chromatography] | ||
[[Category:Protocols]] | [[Category:Protocols]] | ||
Latest revision as of 17:24, 13 April 2017
Contents
Introduction
CHROMATO = colour; of or in colours
GRAPHY = writing...representing...or a process, or art / science working with such a process
Chromatography is a way to analyse what is in a mixture (in this case liquid) - by separation on solid support (in this case the fibers in the paper), and visualization in this case, by a photographic process.
"The mixture flows though a medium and the individual substances that make up the mixture are deposited at different distances from the point of inflow." - from website of Bio-dynamic Society of India
The separation happens as the liquid slowly wets the paper by capillary action -
Those compounds that stick to the paper (medium/ stationary / solid phase), will stay closer to the point of inflow.
Those compounds that prefer the liquid (solvent, mobile phase), it will travel further with the liquid.
This process can just be called paper chromatography. Because we use paper as a support.Because we did this on a circular piece of paper, it is called circular chromatography.
In this case, we took some soil and mud samples we had collected in different water bodies locations, and tried to analyse the humic content of the samples.
This method can be applied to any other liquid mixtures.
For example, this 1953 paper uses butanol-based solvents to separate different sugars, and visualizes the different sugars.
Benedict's reagent may be a nice DIY mix for looking for reducing sugars.
To learn more, here is the Khan Academy's explanation of the Principles of Chromatography.
Methods
This is based on our experience trying out the recommended protocol from the page of BIO-DYNAMIC ASSOCIATION OF INDIA
The original steps from Biodynamic Association of India:
1. A circular filter paper (Whatman #1) with a cylindrical paper wick sitting in a 0.5% solution of silver nitrate is allowed to absorb the solution, which spreads by capillary action, to a certain diameter. 2. The wick is removed and the paper is dried. 3. Meanwhile, the substance to be tested is mixed with a 0.1 to 1% solution of sodium hydroxide and let stand for a period of time. 4. The prepared filter paper is then allowed to absorb this solution and the substance spreads over the paper. 5. When it has spread to a certain distance, the wick is removed and the paper dried. The paper is then exposed to indirect sunlight to let the image develop.
Materials
Chemicals (!! again, this depends on what you want to separate, and what compounds you want to see)
- for visualization - 0.5% solution of silver nitrate (you can find in photography suppliers)
- solvent, mobile phase - 0.1 to 1% solution of sodium hydroxide (NaOH, otherwise known as lye or caustic soda - be careful, because it can burn)
Other materials
- paper (Whatman, or any acid-free thick paper)
- reservoir
- wick (medical cotton, and rolled it into a wick)
- light for development - sunlight is fine unless controlled exposure is wanted
Step by Step
DAY1
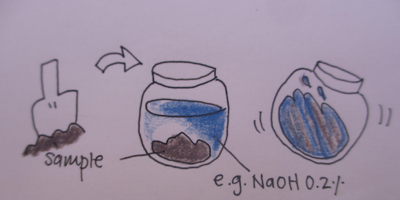
Extraction
This is the step where we take our samples we want to analyse, and extract what is in it into a liquid phase.
Just like taking a crushed piece of garlic, and putting it into water or oil, brings out different flavors in the water and oil, we decided to see what will be extracted from the soil samples when using 2 different solutions.
- 0.2% NaOH in water
- isopropyl alcohol
To learn more, here is a Khan Academy video on extractions.
!!!!Dilute NaOH is used to maximize the recovery of humic substances from soil. For analysis of other compounds, make sure to use appropriate extraction liquid.
- Take one spoon scoop of soil/mud into petridish to mix and extract with NaOH.
- The same scoop of soil/mud into a tube to extract with isoproypl alcohol (it evaporates faster). Here, it may be best to put both in a closeable container, so we can mix and shake the samples.
- Leave the samples overnight at room temperature.
- For better controlled experiments -
- Weigh out equal amounts of soil
- Shake constantly
- Constant temperature during extraction
- Consistent amount of time during extraction
- For better controlled experiments -
DAY2
The next steps needs to happen in a darkroom with a red lamp.
Preparation of Light sensitive paper
- Make the 0.5% silver nitrate solution
- Place 2mL of the 0.5% silver nitrate solution in a clean container
- Place a short wick through the middle of the filter paper
- Place the wick in the silver nitrate
- Wait til the silver nitrate circle is about 1cm from the edge
- Remove the wick
- Let the filter paper dry.
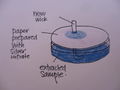
Sample application
- Add a new wick on the prepared paper above
- Take 2mL the liquid part of the samples prepared above, and place in a cleaned container
- Let the sample travel til just before the silver nitrate circle
- Remove wick
- Let the filter dry
Exposure to see the different bands
- Take the filters and expose to indirect light, or in more controlled situations, use a timer and a light set up
- et voila! time for analysis
Analysis of the Chromatogram
The analysis of the final chromatogram - the filter paper - seems more difficult.
Comparison between before and after, different locations, may be easier than to pin-point exactly what is producing the patterns.
See one explanation at the bottom of this webpage.
The different colours we obtain has to be a reflection of the chemical reaction with silver nitrate and whatever compound that is deposited there. (It seems to be copied from here)
For Halides
For example:
- White=Chloride
- Pale Yellow=Bromide
- Yellow=Iodide.
Test for the presence of Carbonate, Hydroxide, Sulfide and Phosphate ions.
For example:
- Pale Green=Carbonate
- Brown=Hydroxide
- Black=Sulfide.
Which method to use
- Bio Crystallisation, copper chloride crystallisation, proteins
- Capillary Dynamolysis, vertical filters, ‘rising pictures’, sugars/ bitter materials
- Bio Chromatography, horizontal circular images, minerals, sugars/ bitter materials
Resources/Links
- Chromatograpy of wine
- Capillary Dynamolysis
- Scandenavian Biodynamics Research
- Academic paper on Capillary Dynamolysis Image Discrimination Using Neural Networks
- Summary of Kolisko's book
- History of Science Group of the Anthroposophical Society in Great Britain
- Khan Academy Principles of Chromatography
- Extraction of Soil Organic Matter
- Types of Paper Chromatography